Is Hcl Covalent or Ionic
Ammonium Chloride is mostly used as a fertilizer but also finds its use in medicine food laboratories batteries etc. Sodium chloride is an ionic compound.

Is Hcl Ionic Or Covalent Techiescientist
Is HCl covalent or ionic bond.

. Therefore HCl is considered a covalent bond. The bond between H and Cl in HCl is formed due to sharing. Conversely if the electronegativity difference is more than 2 it is an ionic bond.
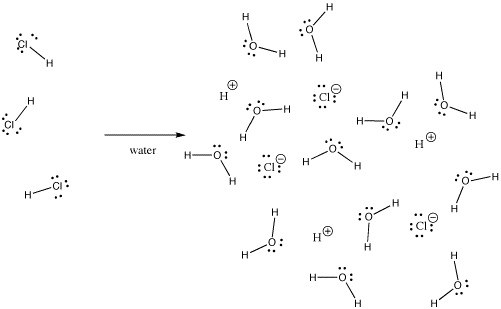
Is the bond in HCl ionic or covalent. Non-ionic compounds are those that have covalent bonds. For instance hydrogen chloride HCl is a gas in which the hydrogen and chlorine are covalently bound but ifHCl is bubbled into water it.
Sodium chloride is an ionic compound. What is not an ionic compound. Because of the difference in electronegativity of H and Cl 09 on Pauling scale it is polar in nature with around 175 ionic character when measured using dipole moment data More.
Many bonds can be covalent in one situation and ionic in another. For instance hydrogen chloride HCl is a gas in which the hydrogen and chlorine are covalently bound but if HCl is bubbled into water it ionizes completely to give the H and Cl- of a hydrochloric acid solution. Synthetically it is produced as a product of the reaction between HCl and ammonia and also as a byproduct of the Solvay process.
Is HCL covalent or ionic. Answer HCl hydrogen chloride is a polar covalent compound. Sodium chloride is an ionic compound.
Hcl is a covalent or ionic polar and nonpolar covalent bondsDONT CLICK THIShttpsrbgymynmw7your queries-topics to be coveredIs HCl Ionic or Covalent. Case in point hydrogen chloride HCl is a gas in which the hydrogen and chlorine are covalently bound however in the event that HCl is risen into water it ionizes totally to give the H and Cl-of a hydrochloric corrosive arrangement. When atoms combine to form molecules the bonds between them may be of two kinds ionic or covalent.
Numerous bonds can be covalent in one circumstance and ionic in another. To tell if HCl Hydrogen chloride is ionic or covalent also called molecular we look at the Periodic Table that and see that H is non-metal and Cl is a no. Many bonds can be covalent in one situation and ionic in another.
As stated in the previous reply calculate the electronegativity difference. Answer 1 of 8. It is a covalent compound made by a single covalent bond between hydrogen and chlorine.
Many bonds can be covalent in one situation and ionic in another. So Is NH 4 Cl ionic or covalent. Hydrochloric acid HCl and carbon tetrachloride CCl 4 form covalent due to sharing of a pair of electrons between two atoms.
HCl is a covalent compound. The bond between H and Cl in HCl is formed due to sharing of electron and is thus covalent. Ammonium chloride is an ionic compound.
Hi Hydrogen chloride is a diatomic molecule consisting of a hydrogen atom H and a chlorine atom Cl connected by a covalent single bond. If the electronegativity is less than 15 then a covalent bond is present. Answer 1 of 15.
For instance hydrogen chloride HCl is a gas in which the hydrogen and chlorine are covalently bound but if HCl is bubbled into water it ionizes completely to give the H and Cl- of a hydrochloric acid solution. Since the chlorine atom is much more electronegative than the hydrogen atom the covalent bond between the two atoms is quite polar. Whereas sodium chloride NaCl and potassium chloride KCl form ionic compounds as they consist of positive and negative.
Is HClO4 Perchloric acid Ionic or CovalentMolecular. To tell if HClO4 is ionic or covalent also called molecular we look at the Periodic Table that.
Is Hcl A Ionic Or Covalent Compound Quora

Is Hcl Ionic Or Covalent Molecular Youtube

Why Is Hydrogen Chloride Hcl A Covalent Compound Why Can T It Be An Ionic Compound Quora

Comments
Post a Comment